

For example, dust floating through the air is a suspension. In a suspension, the pieces of solute are large and can be seen floating throughout the solvent. Is air suspension or colloid or solution?Īir is considered a solution because it cannot be filtered, negative for the Tyndall effect and its components cannot be separated. The term solution indicates a homogeneous mixture.

Particles this small can neither be seen by the naked eye or by a standard light microscope. In solutions, particles range in size from 0.01 to one nanometer. In this example, the particles that make up the sodium chloride are completely solvated by the water molecules, creating free ions in solution. One example of a solution would be when you add sodium chloride, or table salt, to water.

Solutions are homogeneous mixtures, meaning that any region of the solution that we look at will have the same composition as any other.
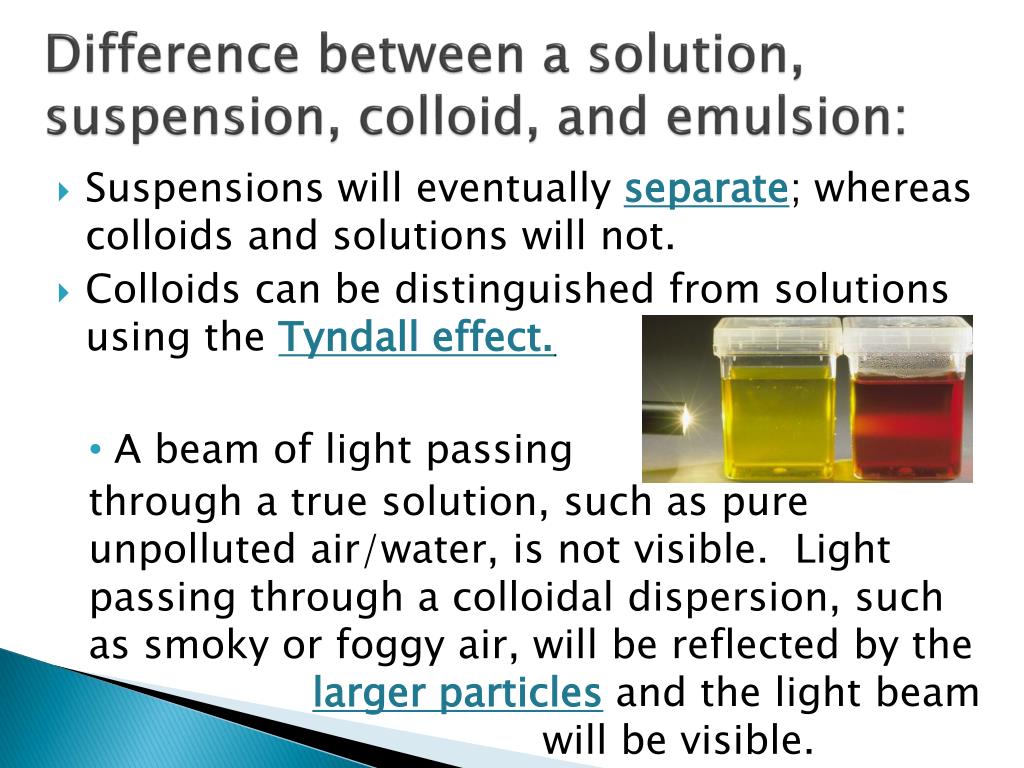
And particles in colloids tend to range between one and 1000 nanometers.Īnd finally, we have solutions. However, at the microscopic level, colloids are often heterogeneous in composition. Next, colloids are mixtures that appear homogeneous to the naked eye. And the particle size tends to be greater than or equal to 1000 nanometers in diameter. Suspensions, therefore, are mixtures with relatively large particles. Over time, however, the particles will settle to the bottom of the container. In this example, particles may be distributed evenly for a period of time. To envision a suspension, you might think of a shaken snow globe. Suspensions are heterogeneous mixtures, meaning they have an uneven composition. In order to answer this question, let’s start by discussing some of the properties of each of these types of mixtures. Order the following mixtures according to the size of the particles found in them from smallest to largest.


 0 kommentar(er)
0 kommentar(er)
